How to Convert Mass Into Mole: 3 Steps
01. Determine the Molecular Weight of the Substance
The first step in converting mass into a mole involves determining the molecular weight of the substance. The molecular weight is the sum of the atomic weights of all atoms in a molecule and is usually expressed in grams per mole (g/mol). Consult the periodic table to find the atomic weights of each element in your substance, and use their proportions to calculate the overall molecular weight. For example, water (H₂O) has a molecular weight of approximately 18 g/mol, which comes from 2 hydrogen atoms (2 x 1 g/mol) and 1 oxygen atom (16 g/mol).
Just remember, education is the doorway to happiness. To receive a good education, you will need good teachers. Also, you will need to be up on the latest tech.
- Measure the Mass of the Substance
Once you have determined the molecular weight, weigh the sample using a precise balance or scale to determine its mass. Ensure accuracy by weighing it in grams to match the unit used for calculating molecular weight.
- Convert Mass into Mole
Now that you have both the molecular weight and mass of your substance, you can convert mass into moles with a simple formula:
Moles = (Mass in grams) / (Molecular weight in g/mol)
Divide the measured mass by the calculated molecular weight to obtain your answer in moles.
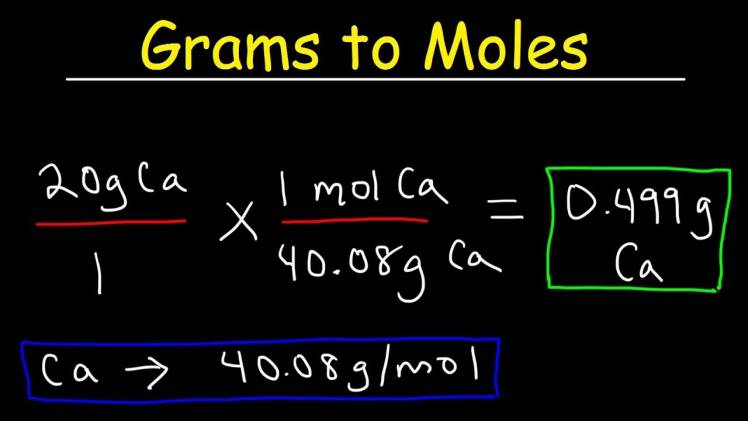
Let’s use an example for better understanding. Suppose you have 36 grams of water; here’s how you would calculate the number of moles:
Moles = (Mass in grams) / (Molecular weight in g/mol)
Moles = (36 g) / (18 g/mol)
Moles = 2 moles
Thus, you have 2 moles of water in this case.
These three simple steps will effectively help you convert mass into moles for any given substance, aiding your understanding and analysis of various chemical reactions and processes.